Below are some frequently asked questions about IONIS-HTTRx, which recently completed a successful clinical trial for safety. Click here for full announcement.
What’s the big news?
On December 11, 2017, Ionis Pharmaceuticals announced the results of a small clinical trial to test the safety of a new HD drug called IONIS-HTTRx. It is the very first huntingtin-lowering drug.
What were the results?
In this early trial of about 40 participants in Europe and Canada, the drug was safe (no participants had a dangerous medical event) and tolerable (no dangerous side effects). It lowered levels of the harmful protein that causes HD.
How excited should I be about this?
HD organizations, researchers, and the media are excited because it is the first time that a drug created to alter the genetics of a brain disease has proven safe in a human clinical trial. This is good news for HD and many other brain diseases, but there are more steps required to test and approve this drug.
Could this be the cure we have been waiting for?
Although we now know it is safe and has an impact in the human body, this Phase 1b/2a trial was very small and it was only designed to test IONIS-HTTRx for safety and huntingtin-lowering. We do not know yet whether the drug will improve symptoms or slow the disease.
What happens next?
Roche Pharmaceuticals will take over the project. A Phase III trial will be conducted globally, including sites in the US. There is not yet information available about where and when this will occur.
Is the trial open to everyone?
All clinical trials have eligibility criteria, meaning that whether a person can participate is based on factors like age, disease progression, symptoms, and other medical conditions. Eligibility details have not yet been provided by Roche, but they will be posted on HDTrialfinder.org and clinicaltrials.gov as soon as they are available.
Is it true you need to get a spinal tap for this drug?
Yes. In this trial, the drug is delivered monthly through a needle to the spinal column. This is routinely done during labor and delivery and for treatment of some cancers.
Will this work for Juvenile Huntington’s Disease too?
It is likely that initial clinical trials will focus on adults, and that separate trials would be required to test the drug in children. In theory, however, this therapy could also lower huntingtin in JHD.
How do I get myself/my loved one into the trial?
The next phase of the trial is not yet underway, and recruitment is a carefully planned process. When a trial begins, doctors at open study sites speak with their patients who might be a good fit. We do not yet know where the trial will occur, who is eligible to participate, or who to contact about participation, but this information will be shared as soon as it becomes available at HDTrialfinder.org.
What can I do right now?
The best ways to stay involved in HD clinical research and be seen by clinician-researchers likely to run trials are to (1) join Enroll-HD, a worldwide observational HD study open to anyone with HD or at risk, and (2) stay updated about open trials through HDSA’s trial-matching service at HDTrialfinder.org.
Where can I read more?
Check out this HDBuzz article with further details. https://en.hdbuzz.net/249
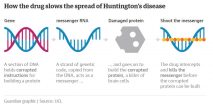
Click on the graphic below to see how the drug slows the spread of Huntington’s disease: